Abstract
Introduction: Recombinant factor IX Fc fusion protein (rFIXFc) was the first extended half-life FIX product approved in the United States to treat children and adults with hemophilia B. Long-term data from clinical trials have demonstrated the safety and efficacy of rFIXFc as well as an extended dosing interval (once weekly or every 10‒14 days based on individual needs); however, real-world data are limited (Wang et al. Haemophilia, 2018; Buckley et al. AMCP NEXUS, 2015). We therefore performed a retrospective chart review to further understand the clinical experience and outcomes associated with real-world treatment of hemophilia B with rFIXFc.
Methods: This retrospective chart review is being conducted at 6 sites across different regions of the United States and aims to include 70 patient charts. Data entry for 43 patient charts has been completed to date (cutoff: June 29, 2018). Data collection is ongoing.
Inclusion criteria were diagnosis of hemophilia B and receipt of rFIXFc for ≥6 months. Subjects with other coagulation disorders or any record of positive FIX inhibitor titers were excluded. De-identified subject-level data were transcribed onto anonymous electronic case report forms. Endpoints included changes in FIX therapy dosing interval, factor consumption, bleed control, and patient adherence before and after rFIXFc initiation. Descriptive statistics were used to summarize the results.
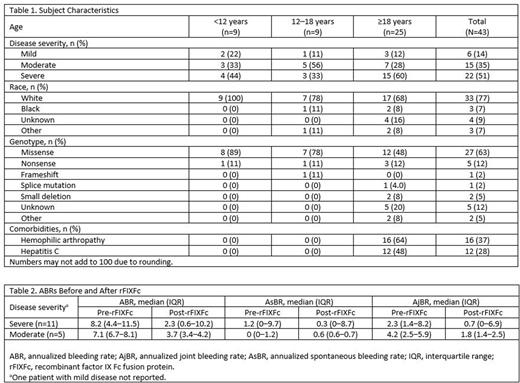
Results: For the 43 charts available for analysis, the duration of follow-up receiving rFIXFc ranged from 0.5 to 3.7 years. Of these, 58% of subjects (25/43) were >18 years of age, 77% (33/43) were of white race, and 51% (22/43) had severe hemophilia B (Table 1). The most common genotype was missense, occurring in 63% of subjects (27/43). Among subjects with comorbidity, 37% reported hemophilic arthropathy (16/43) and 28% had hepatitis C (12/43).
All 22 subjects with severe hemophilia B were treated with rFIXFc prophylactically compared with 9/15 moderate and 3/6 mild cases. From the data collected thus far, 94% of prophylaxis subjects were on a dosing interval of weekly or longer (every 7 days, n=20; every 10 days, n=3; and every 14 days, n=9). The total weekly dose before and after switching to rFIXFc prophylaxis were available for 20 subjects. Of the 12 adults (9 severe, 2 moderate, and 1 mild), the median weekly factor consumption decreased from 111 IU/kg to 52.5 IU/kg. A similar pattern was observed for subjects who were 12-18 years of age (n=4). For subjects <12 years of age (n=4), 2 had reduced weekly consumption after switching to rFIXFc, whereas 2 (on plasma-derived product pre-rFIXFc) had an increase in weekly consumption.
The annualized bleeding rates (ABRs), annualized spontaneous bleeding rates (AsBRs), and annualized joint bleeding rates (AjBRs) were available for 17 subjects treated with prophylaxis regimens pre- and post-rFIXFc (Table 2). Of these subjects, 11 had severe, 5 had moderate, and 1 had mild hemophilia B. Among 11 subjects with severe hemophilia B, median (interquartile range) ABRs decreased from 8.2 (4.4‒11.5) to 2.3 (0.6‒10.2), AsBR from 1.2 (0‒9.7) to 0.3 (0‒8.7), and AjBR from 2.3 (1.4‒8.2) to 0.7 (0‒6.9) before and after rFIXFc treatment. Subjects with moderate disease had a similar pattern (Table 2).
The most common reason for switching to rFIXFc was to reduce the burden associated with therapy (21/43, 49%). No rationale for switching was documented in 40% (17/43) of subjects, and 7% (3/43) switched due to lack of efficacy with previous treatment. The other reasons, including difficult venous access, lack of adherence, and failure to reach desired trough were mentioned by <5% of subjects. No subject reported adherence issues while on rFIXFc.
Conclusions: This real-world study of rFIXFc demonstrates improved bleed control, reduced overall consumption, and reduced frequency of injection for subjects with moderate and severe hemophilia B. The data also show that rFIXFc provides an opportunity to tailor dosing and improve adherence. These results echo the findings of the pivotal trials and add to the pool of evidence supporting rFIXFc in the treatment of hemophilia B. These data also reflect the use of rFIXFc for mild hemophilia patients in the real-world setting.
Shapiro:Kedrion Biopharma: Consultancy, Research Funding; Novo Nordisk: Membership on an entity's Board of Directors or advisory committees, Research Funding; Bioverativ, a Sanofi Company: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Prometic Life Sciences: Consultancy, Research Funding; Shire: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Genetech: Membership on an entity's Board of Directors or advisory committees, Research Funding, Speakers Bureau; Bayer Healthcare: Other: International Network of Pediatric Hemophilia; BioMarin: Research Funding; Bio Products Laboratory: Consultancy; Octapharma: Research Funding; OPKO: Research Funding; Sangamo Biosciences: Consultancy; Daiichi Sankyo: Research Funding. Chaudhury:Bioverativ, a Sanofi Company: Consultancy, Research Funding; Bayer: Membership on an entity's Board of Directors or advisory committees. Jain:Bioverativ: Employment. Tsao:Bioverativ: Employment. Barnowski:Bioverativ, a Sanofi Company: Employment. Feng:Bioverativ: Employment. Quon:Shire: Speakers Bureau; Genetech: Consultancy, Speakers Bureau; NovoNordisk: Consultancy, Speakers Bureau; Bayer: Consultancy; Octapharma: Consultancy; Bioverativ, a Sanofi Company: Speakers Bureau.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal